Abstract
Background
CAR T-cell therapies directed against CD19 (CAR19) are used to treat relapsed/refractory (R/R) B-acute lymphoblastic leukemia (B-ALL) and show high complete remission (CR) rates compared to salvage chemotherapy. However, subsets of patients do not respond or relapse after initial CR. While previous work has demonstrated the importance of CAR T cell immunophenotype and vector integration site in long term CAR19 responders, there are currently no clinically applicable predictors of response. Given the importance of the B-ALL tumor microenvironment in mediating chemoresistance, inflammatory signaling and recruitment of immune effectors to the leukemic bone marrow, we hypothesized that the pre-CAR19 infusion bone marrow biopsy immune microenvironment may predict CAR19 responses.
Methods
Patients treated with CAR19 for R/R B-ALL from 2012-2017 were retrospectively identified. Marrow core biopsies were collected immediately prior to CAR19 infusion (pre-CAR) and at one month post-infusion (post-CAR). Bone marrow biopsy slides immunohistochemically stained for CD3 were digitally scanned, analyzed algorithmically for T-cell infiltration and assessed qualitatively for interstitial T cell aggregates of >5 cells (Leica Aperio ImageScope). B-ALL involvement was determined by bone marrow aspirate counts and minimal residual disease (MRD) flow cytometry. Targeted RNA sequencing-based gene expression profiling (EdgeSeq Immuno-Oncology Panel, HTG Diagnostics) was performed on pre- and post-treatment biopsies, with differential expression assessed by DESeq2 within HTG Reveal software, and further analyses conducted using Metascape.
Results
We identified 143 bone marrow core biopsies (84 pre-treatment, 59 post-treatment) from 84 R/R B-ALL patients treated with CAR19. Pre-CAR B-ALL marrow involvement ranged from 0-95% (median: 67.5%), and the dataset included patients with relapses occurring between 1-33 months following CAR therapy (median: 6 months).
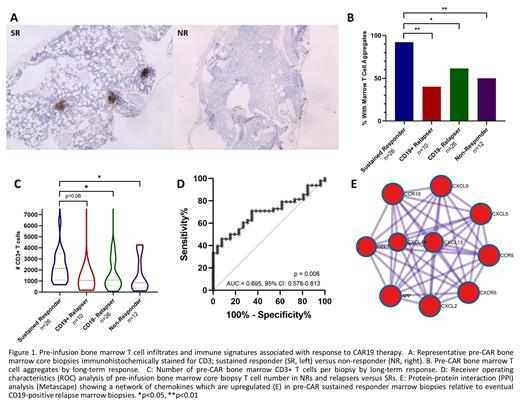
Pre-CAR CD3+ T cell aggregates were significantly increased in CAR19 Sustained Responders (SR) compared to Non-Responders (NR) (92.3% versus 50%, p=0.007) and eventual relapses (CD19+: 40%, p= 0.002 and CD19-: 61.5%, p=0.019) (Figure 1A-B). A lack of T cell aggregates in pre-CAR marrow biopsies showed a sensitivity of 45.8% and a specificity of 92.3% in distinguishing NRs and eventual relapses from SRs. In one month post-CAR marrow biopsies, T cell aggregates continued to show higher frequency in SRs compared to eventual CD19+ relapses (100% versus 42.9%, p=0.007) and NRs (50%, p=0.04).
Pre-CAR marrow T cell infiltration was also significantly greater in SRs compared to NRs (2146 CD3+ T cells per biopsy versus 850, p=0.038) and eventual CD19- relapses (1086, p=0.022), and trended toward a significant increase compared to eventual CD19+ relapses (1056, p=0.063) (Figure 1C). Pre-CAR marrow T cells of < 920 per biopsy showed a sensitivity of 45.8% and a specificity of 92.3% in distinguishing NRs and relapses from SRs (Figure 1D).
RNAseq differential gene expression analysis of pre-CAR marrow biopsies revealed a chemokine axis of CXCL9, CXCL13, CXCR5, CXCL5, CXCL2 and CCL1 in SR that was deficient in patients with eventual relapse (Figure 1E). By contrast, NRs and eventual relapses showed a pre-CAR transcriptional signature reflecting Th2 (IL5RA, IL13) and Th17 (IL17A, IL23R) skewing, in addition to higher IL2, IFNG and IL6 transcripts.
Conclusion
These immunohistochemical and transcriptional findings support a pivotal role for the peri-infusion bone marrow microenvironment in long-term responses to CAR19 therapy in B-ALL. A deficiency of marrow T cell aggregates and marrow-infiltrating T cells pre-CAR shows high specificity in distinguishing NRs and eventual relapses from SRs to CAR19, and marrow T cell aggregates are a persistent feature of SRs at one month post-CAR infusion. SR to CAR19 is also associated with elevated marrow expression of a suite of immune chemoattractants which may enhance the ability of infused CAR T cells and other effectors to infiltrate the B-ALL microenvironment. Combined assessment of bone marrow T cell infiltrates and transcriptional profiling could be used to improve the sensitivity and specificity of predictive algorithms for responses to CAR19 therapy.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal